近日,我院劳动卫生与环境卫生研究团队在研究纳米氧化铁细胞氧化还原酶系稳态方面研究取得新进展,为吸入钢铁粉尘等含铁颗粒物的毒性损伤预防提供科学依据。相关研究成果以“Intracellular GSH/GST antioxidants system change as an earlier biomarker for toxicity evaluation of iron oxide nanoparticles”为题,发表在期刊NanoImpact(IF=5.316)(DOI 10.1016/j.impact.2021.100338)上。
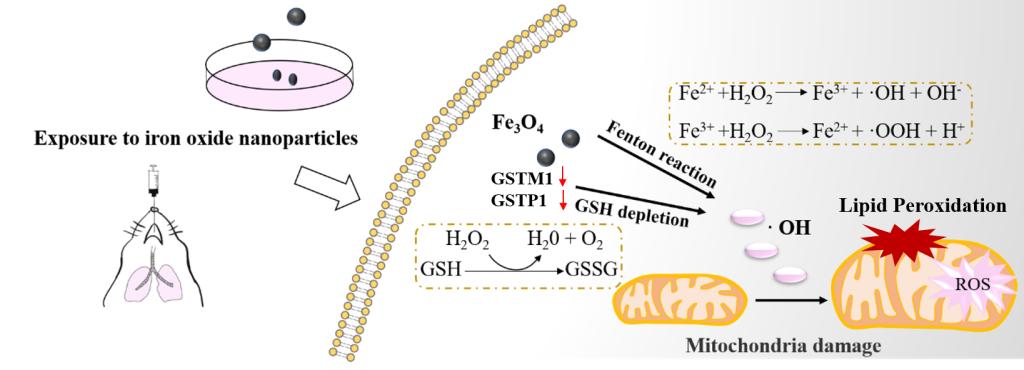
交通尾气、钢铁锻造或焊接过程中产生的氧化铁纳米颗粒作为一种潜在的环境污染源,可经由人体呼吸过程进入体内,并通过引起机体持续的氧化应激来加剧对机体的损伤。谷胱甘肽和谷胱甘肽-S-转移酶系统是机体防御外源性有毒物质引起氧化应激并造成急慢性毒性的“先锋”。谷胱甘肽和谷胱甘肽-S-转移酶对抗氧化应激相关信号通路的调节以及机体内谷胱甘肽水平的动态平衡在机体对众多环境毒素的解毒过程中起着核心作用。因此,在唐敬龙老师的指导下,本课题组通过纳米氧化铁(Fe2O3和Fe3O4)在细胞内和动物体内的两种急性暴露实验,以及测定暴露后谷胱甘肽水平和谷胱甘肽-S-转移酶活性,来探究谷胱甘肽和谷胱甘肽-S-转移酶在机体抵抗纳米氧化铁颗粒引起的氧化应激损伤中发挥的作用。
经研究发现,较低浓度的Fe3O4纳米粒子(≤100μg/ml)可消耗谷胱甘肽水平同时降低谷胱甘肽-S-转移酶的活性,对人支气管上皮细胞的毒性较大。且Fe3O4本身的酶催化活性可能会抑制谷胱甘肽-S-转移酶M和P(GSTM1和GSTP1)的活性位点,并减少GSTM1和GSTP1的表达从而导致细胞内谷胱甘肽的消耗。而且谷胱甘肽的消耗和谷胱甘肽-S-转移酶活性的降低可能与进一步的脂质过氧化反应有关,可作为评价氧化铁纳米颗粒毒性的早期生物标志物,相应的干预措施可能对预防氧化铁纳米颗粒引起的呼吸暴露损伤有效。51cg今日吃瓜热门大瓜唐敬龙老师和天津医科大学张明亮老师为本文共同通讯作者,硕士研究生张婉君、高金玲(为本文共同第一作者。上述研究得到了国家自然科学基金(81872651、92043202、91943301)和国家科技部重点研发计划(2017YFC1600200)的支持。
Recently, the research team of occupational and environmental health of our school has made new progress in the study of early biomarkers of the toxic effect of iron oxide nanoparticles (mainly Fe3O4 particles) on human body. The related research results were published in the journal NanoImpact (IF=5.316) (DOI 10.1016/j.impact.2021.100338) under the title of "Intracellular GSH/GST antioxidants system change as an earlier biomarker for toxicity evaluation of iron oxide nanoparticles".
Iron oxide nanoparticles stemmed from traffic exhaust, steel manufacturing, or welding as a potential environmental pollution can lead to adverse respiratory outcomes and aggravate the risk of chronic health conditions via persistent oxidative stress. Glutathione (GSH) and glutathione-S-transferases (GSTs) are two frontlines of cellular defense against both acute and chronic toxicity of xenobiotics-induced oxidative stress. The contribution of GSH and GST enzymes to signaling pathways and the regulation of GSH homeostasis play a central role in the detoxification of numerous environmental toxins and impurities.Based on this, directed by Prof. Jinglong Tang, the research team conducted two kinds of acute exposure experiments of iron oxide (Fe2O3 and Fe3O4) nanoparticles in cells and in vivo, and evaluated the GSH levels and GST activity after exposure to assess the role of GSH/GST antioxidants system in the body's resistance caused by iron oxide nanoparticles.
It was found that Fe3O4 nanoparticles at lower concentrations (≤100 μg/ml) seem to be more toxic to the human bronchial epithelial cells as their consumption of GSH and decrease of GST activity. The catalysis activity of Fe3O4 nanoparticles per se may contribute to the intracellular GSH consumption along with inhibition of glutathione-S-transferase class M and P (GSTM1 and GSTP1) active site and expression decrease of GSTM1 and GSTP1. Accordingly, the GSH consumption and decrease in GST activity directed to the further lipid peroxidation regarded as an earlier marker for toxicity evaluation of iron oxide nanoparticles, and relevant intervention may be effective for prevention of respiratory exposure induced damage from iron oxide nanoparticles. Prof. Jinglong Tang and Mingliang Zhang were co-supervised the study. Graduate students Wanjun Zhang, Jinling Gao (Now at Department of Infection Management Service, Dushu Lake Hospital Affiliated of Soochow University) were the co-first authors. The above research was supported by the National Science Foundation of China (81872651, 92043202, and 91943301) and the Ministry of Science and Technology of China (National Key Research and Development Project 2017YFC1600200).