近日,我院劳动卫生与环境卫生系晋小婷教授团队在研究环境浓度颗粒物暴露与线粒体动力学及关键理化性质方面取得重要成果,相关研究成果以“Surface charge-dependent mitochondrial response to similar intracellular nanoparticle contents at sublethal dosages”和“Airborne particulate matters induce thrombopoiesis from megakaryocytes through regulating mitochondrial oxidative phosphorylation”为题均发表于环境毒理学TOP一区期刊Particle and fibre toxicology(影响因子:9.402;DOI分别为10.1186/s12989-021-00429-8和10.1186/s12989-021-00411-4)。
该系列研究通过评价线粒体特征和相应标志物,阐明了环境浓度颗粒物与线粒体动力学的相关性,揭示了颗粒物引发线粒体动态紊乱的关键理化性质。一方面,在相似的内暴露剂量下,不同涂层和直径的纳米颗粒对线粒体反应产生不同程度的影响,包括管状线粒体减少、线粒体受损、活性氧增加和三磷酸腺苷减少。聚类分析、双向方差分析和多元线性回归分析表明,纳米颗粒的表面性质是线粒体反应的主要决定因素,同时提示复合物V是线粒体对亚致死剂量纳米颗粒的敏感指标。另一方面,研究成果首次聚焦颗粒物靶器官肺组织的造血免疫功能,聚焦造血干细胞分化的关键窗口期,即巨核细胞分化为血小板过程,揭示了不同粒径不同采样膜的大气颗粒物对巨核细胞成熟以及分化为血小板过程的影响;进一步基于差异蛋白质组学结果以及抑制剂实验,发现线粒体氧化磷酸化过程为颗粒物引发血小板生成的关键通路,且线粒体呼吸链电子传递过程以及伴随产生的线粒体活性氧可能共同调控巨核细胞成熟。这些发现首次阐明环境浓度颗粒物引发线粒体动力学紊乱的关键理化性质,并揭示线粒体呼吸链功能在颗粒物引发肺造血分化关键窗口期巨核细胞成熟分化过程的贡献。为雾霾颗粒物引发心血管疾病风险机制阐释提供了新的视角和重要科学数据。
晋小婷教授为两篇文章的第一作者,硕士研究生于海艺和于红燕分别为两篇文章的第二作者。本研究合作单位为中国科学院生态环境研究中心。该课题得到国家自然科学基金(Nos. 91943301, 21976114, 92043202和21976189)和山东省泰山青年学者基金(tsqn201909101)的支持。
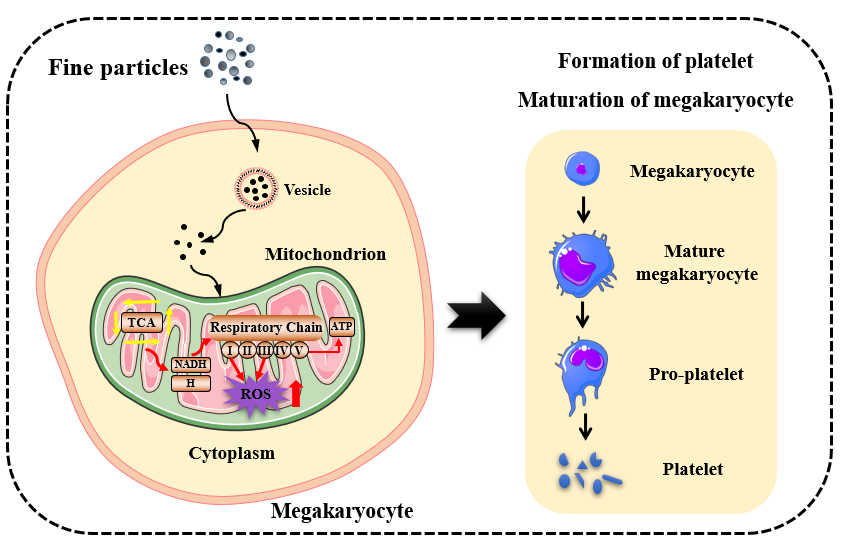
图. 大气颗粒物暴露对巨核细胞成熟分化的影响及潜在机制。(图片来自DOI: 10.1186/s12989-021-00411-4)
The research team from the department of occupational and environmental health has made important progress in studying the mitochondrial dynamics upon particulate matter exposure and uncovering the key physicochemical properties
Recently, professor Xiaoting Jin’s group from the department of occupational and environmental health has achieved important results in studying the mitochondrial dynamics exposure to ambient concentrations of particulate matter (PM2.5) and the key physicochemical properties. Relevant results have been published in Particle and Fibre Toxicology (TOP 1 journal in environmental toxicology, Impact Factor: 9.402) under the title “Surface charge-dependent mitochondrial response to similar intracellular nanoparticle contents at sublethal dosages” and “Airborne particulate matters induce thrombopoiesis from megakaryocytes through regulating mitochondrial oxidative phosphorylation”. (DOI 10.1186/s12989-021-00429-8 and 10.1186/s12989-021-00411-4, respectively).
The studies elucidated the correlation between ambient concentrations of PM2.5 and mitochondrial dynamics by evaluating mitochondrial characteristics and corresponding biomarkers, and revealed the key physicochemical properties of PM2.5-induced disturbances in mitochondrial dynamics. On the one hand, at similar internal exposure doses, nanoparticles with different coatings and diameters exerted different degrees of effects on mitochondrial responses, including reduced tubular mitochondria, impaired mitochondria, increased reactive oxygen species, and reduced ATP. Cluster analysis, two-way ANOVA and multiple linear regression analysis showed that the surface properties of nanoparticles were the main determinants of mitochondrial response, together with suggesting that complex V was a sensitive indicator of mitochondrial response to sublethal doses of nanoparticles. On the other hand, the research for the first time focused on the hematopoietic immune function of lung tissue (the target organ of PM2.5) and the crucial process of hematopoietic stem cell differentiation (i.e., the differentiation of megakaryocytes into platelets), revealing the effect of atmospheric PM2.5 from different particle sizes and sampling membranes on the maturation of megakaryocytes and their differentiation into platelets. Furthermore, based on differential proteomic results and the addition of inhibitors, the mitochondrial oxidative phosphorylation was found to be the key pathway for PM-triggered platelet production, and the mitochondrial respiratory chain electron transport process and accompanying mitochondrial reactive oxygen species may jointly regulate the maturation of megakaryocytes. These findings were the first to elucidate the key physicochemical properties of mitochondrial kinetic disorders triggered by ambient concentrations of PM2.5 and to reveal the contribution of the mitochondrial respiratory chain in the maturation and differentiation of megakaryocytes. These results provided a new perspective and important scientific evidence for the elucidation of the mechanisms of cardiovascular diseases induced by haze particulate matter.
Prof. Xiaoting Jin is the first author of two articles, and postgraduate students Haiyi Yu and Hongyan Yu are the second authors of two articles, respectively. The collaborator of this study is the Research Center for Eco-Environmental Sciences, the Chinese Academy of Sciences. This work was financially supported by the National Natural Science Foundation of China (No. 91943301, 21976114, 92043202, and 21976189) and the Taishan Youth Scholar Fund of Shandong Province (No. tsqn201909101).